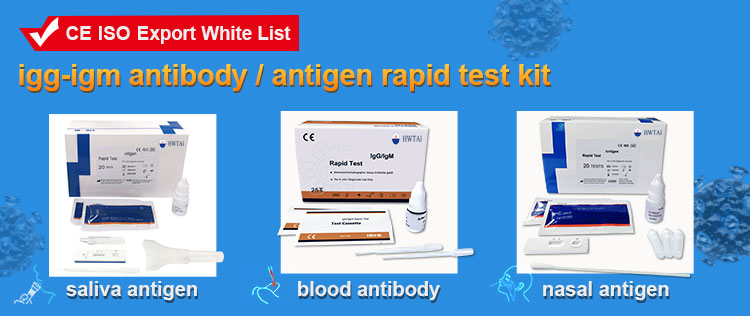
The Strep A Rapid Test Device (Throat Swab) is a qualitative, lateral flow immunoassay for the detection of Strep A carbohydrate antigen in a throat swab. In this test, antibody specific to Strep A carbohydrate antigen is coated on the test line region of the test. During testing, the extracted throat swab specimen reacts with an antibody to Strep A that is coated onto particles. The mixture migrates up the membrane to react with the antibody to Strep A on the membrane and generate a color line in the test line region. The presence of this color line in the test line region indicates a positive result, while its absence indicates a negative result. To serve as a procedural control, a colored line will always appear in the control line region, indicating that proper volume of specimen has been added and membrane wicking has occurred.

Explain :
| The Strep A Rapid test Device (Throat Swab) is a rapid chromatographic immunoassay for the qualitative detection of Strep A antigen from throat Swab specimens to aid in the diagnosis of Group A Streptococcal infection. |
The Strep A Rapid test Device (Throat Swab) is a qualitative, lateral flow immunoassay for the detection of Strep A carbohydrate antigen in a throat swab. In this test, antibody specific to Strep A carbohydrate antigen is coated on the test line region of the test. During testing, the extracted throat swab specimen reacts with an antibody to Strep A that is coated onto particles. The mixture migrates up the membrane to react with the antibody to Strep A on the membrane and generate a color line in the test line region. The presence of this color line in the test line region indicates a positive result, while its absence indicates a negative result. To serve as a procedural control, a colored line will always appear in the control line region, indicating that proper volume of specimen has been added and membrane wicking has occurred.
Kit Components · Test devices· Sterile swabs· Strep A Reagent A(1M Sodium Nitrite)· Strep A Positive control(Non-viable Strep A; 0.09% NaN3)· Workstation· Test Tubes· Strep A Reagent B(0.4M Acetic Acid)· Strep A Negative control(Non-viable Strep C; 0.09% NaN3)· Dropper tips· Package insert |

Infectious disease Test | |||
Product | Item No. | Description | Specimen |
M.Tuberculosis | ITB-302 | TB Ab (lgG) test cassette | P/S |
Helicobacter Pylori | IHP-602 | HP Ag test cassette | Feces |
IHP-302 | HP Ab test cassette | P/S | |
Rota/Adenovirus | IRA-622 | Rota/Adeno Ag test cassette | Feces |
Rotavirus Group A | IRO-602 | Rota Ag test cassette | Feces |
HbsAg | IHBsg-402 | Hepatitis B Surface Ag test | WB/S/P |
HbsAb | Hepatitis B Surface Ag test | WB/S/P | |
HCV | IHC-402 | Hepatitis C Virus | WB/S/P |
Malaria | IMA-402 | Malaria pf Test Cassette | WB |
IMA-422 | Malaria pf/pv test cassette | WB | |
IMA-432 | Malaria pf/pan test cassette | WB | |
TORcH | ITH-352 | TORcH IgM test cassette | S/P |
Strep A | ISA-502 | Strep A test | throat swab |
Dengue | IDE-422 | Dengue IgG/IgM | WB/S/P |
IDE-402 | Dengue NS1 | WB/S/P | |
IDE-432 | Dengue IgG/IgM/ NS1 combo | WB/S/P | |
Chagas | ICS-402 | Chagas antibody test cassette | WB/S/P |
Chikungunya | ICA-402 | Chikungunya ab (IgM) test cassette | WB/S/P |
Filariasis | IFI-422 | Filariasis IgG/ IgM test cassette | WB/S/P |
Typhoid | ITY-422 | Typhoid IgG/IgM test cassette | WB/S/P |
Toxoplasma | ITO-422 | Toxoplasma IgG/IgM test cassette | WB/S/P |
Leishmania | ILE-402 | Leishmania IgG test cassette | WB/S/P |


Contact: Neo
Phone: 008615867460640
E-mail: Info@Hwtai.com
Whatsapp:008615867460640
Add: Building 2, Xinmao Qilu Science Technology Industrial Park, Tianqiao District, Jinan City, Shandong Province,China.
We chat
